Moving Beyond COVID-19: Vaccines and Other Policy Considerations in Latin America
Download the Report
Introduction
Parts of Latin America and the Caribbean have become COVID-19 hot spots. With 8 percent of the world’s population, the region accounts for nearly one-third of all COVID-19 infections and deaths as of late November.1Author’s calculations. Source: Johns Hopkins University. 2020. “COVID-19 Dashboard.” Coronavirus Resource Center. Accessed November 23, 2020. https://coronavirus.jhu.edu/map.html. Although new infections have slowed on a regional level, ongoing second or third pandemic waves across Europe and the United States could be a sign of things to come.2Author’s calculations. Source: Johns Hopkins University. 2020. “COVID-19 Dashboard.” Coronavirus Resource Center. Accessed November 23, 2020https://coronavirus.jhu.edu/map.html. Globally and in Latin America, life will likely not return to normal without an effective vaccine. Naturally acquired herd immunity appears elusive.
In this context, herculean R&D efforts have accelerated the race for COVID-19 vaccines at record pace. At least nine candidates are undergoing Phase III clinical trials at the time of writing, including two reporting more than 90 percent efficiency.3Denise Grady, “Early Data Show Moderna’s Coronavirus Vaccine Is 94.5% Effective,” New York Times, last updated November 20, 2020, https://www.nytimes.com/2020/11/16/health/Covid-moderna-vaccine.html. Despite the considerable progress, the U.S. Food and Drug Administration (FDA) had not approved a vaccine for nonemergency use as of November. Uncertainty and questions have arisen beyond vaccine efficacy and risks. How can countries and populations quickly and safely access and administer vaccines? Can it be done in a fair and equitable way? When will this happen and at what cost? Will there be enough for everyone? Similar questions also apply to potential COVID-19 therapeutics, test solutions, personal protective equipment (PPE), and ventilators, as witnessed early in the pandemic.
As these under-studied questions come to the fore globally, Latin America must plan ahead with added urgency. The region may face vaccine and related hurdles at a greater scale due to financial, technical, and logistic constraints. Policy decisions and actions today will affect health outcomes in the coming months and years, either curtailing or widening the region’s capacity gaps.
In particular, preparation in nine areas will determine regional success in immediate vaccine planning and longer-term public health. The following pages outline each of these areas, including relevant recent developments, major challenges, and potential remedies. Through this analysis and a resulting set of eight cross-cutting recommendations, this practical policy primer aims to inform regional governments and others critical to mitigating the health effects of COVID-19 in order to better prepare the region for the next set of pandemic-related challenges. The historic magnitude of the ongoing pandemic requires a whole-of-society approach: collaboration among relevant stakeholders from governments, the private sector, multilateral organizations, and civil society will be indispensable.
Conclusions and Recommendations
Download the Report
Policy decisions and actions today will affect health outcomes in the coming months and years, either curtailing or widening [Latin America’s] capacity gaps.
Vaccine production and acquisition
For many Latin American countries, a two-pronged approach—bilateral and multilateral partnerships—is needed to acquire a sufficient amount of vaccines for their citizens. Bilaterally, countries including Argentina, Brazil, Chile, Mexico, and Peru have secured more than two hundred and eighty-six million potential doses through production or purchase agreements with various pharmaceutical companies.4Author’s calculations. Sources: Reuters staff, “Brazil’s Bolsonaro orders $360 million to be set aside for AstraZeneca coronavirus vaccine,” Reuters, last updated August 6, 2020, https://www.reuters.com/article/us-health-coronavirus-brazil/brazils-bolsonaro-orders-360-million-to-be-set-aside-for-astrazeneca-coronavirus-vaccine-idUSKCN2523BH. Reuters staff, “Argentina agrees deal for 22 million doses of AstraZeneca-Oxford COVID-19 vaccine,” Reuters, last updated November 7, 2020, https://www.reuters.com/article/us-health-coronavirus-argentina-astrazen-idUSKBN27N0UQ?utm_campaign=trueAnthem%3A+Trending+Content&utm_medium=trueAnthem&utm_source=facebook. Luisa Horwitz, “Timeline: Latin America’s Race for a COVID-19 Vaccine,” AS/COA, October 27, 2020, https://www.as-coa.org/articles/timeline-latin-americas-race-covid-19-vaccine. The region’s active participation in advanced clinical trials of vaccines has helped strengthen negotiation positions for access.5Jude Webber, “Mexico Uses Human Trials As Path To Secure Future Covid-19 Vaccines,” Financial Times, October 26, 2020, https://www.ft.com/content/8beceb2f-14b1-4071-9283-0307159feff2. On the multilateral front, twenty-two Latin American and Caribbean countries will obtain millions of effective doses through the COVAX Facility, a global initiative co-led by the Coalition for Epidemic Preparedness Innovations (CEPI), Gavi, and the World Health Organization (WHO).6COVAX, “Commitment agreements,” Gavi, last updated October 29, 2020, https://www.gavi.org/sites/default/files/covid/pr/COVAX_CA_COIP_List_COVAX_PR_29-10.pdf. Working with a diverse portfolio of vaccine candidates through multiple procurement sources is indispensable because at least some current candidates will fall short of desired safety or efficacy requirements—a normal and historically common outcome. The region could need well over seven hundred million functioning doses to reach a theoretical threshold of herd immunity (60 percent) through vaccination.7Author’s calculations. Considering a theoretical herd immunity threshold of 60 percent, a regional population of six hundred and fifty million, and a two-shot vaccine. Sources: Meghie Rodrigues, “Brazil city ‘might have reached herd immunity,’” Gavi, last updated October 16, 2020, https://www.gavi.org/vaccineswork/brazil-city-might-have-reached-herd-immunity. Center for Disease Control and Prevention. 2020. “Frequently Asked Questions about COVID-19 Vaccination.” Last updated November 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html.
There are two ways to acquire vaccines: local production and trade. Each comes with its own set of challenges.
There are two ways to acquire vaccines: local production and trade. Each comes with its own set of challenges.
- Local Production
Producing COVID-19 vaccines is an unprecedented global challenge, prompting the world to double its production over twelve to eighteen months.8Topher Spiro and Zeke Emanuel, “A Comprehensive COVID-19 Vaccine Plan,” Center for American Progress, July 28, 2020, https://www.americanprogress.org/issues/healthcare/reports/2020/07/28/488196/comprehensive-covid-19-vaccine-plan/. Rep. Raja Krishnamoorthi, “Trust and transparency are necessary to make COVID-19 vaccine successful,” Hill, October 23, 2020, https://thehill.com/blogs/congress-blog/healthcare/522400-trust-and-transparency-are-necessary-to-make-covid-19-vaccine. Latin America is taking part in these global efforts. For instance, Argentina, Brazil, and Mexico have made arrangements with AstraZeneca and the University of Oxford to supply up to four hundred million doses of the AZD1222 vaccine to regional markets.9Raul Cortes and Daina Beth Solomon, “AstraZeneca set to start making 400 million COVID-19 vaccines for Latam early in 2021,” Reuters, August 13, 2020, https://uk.reuters.com/article/uk-health-coronavirus-latam-vaccine-idUKKCN2592N3. The vaccines will be produced in an mAbxience plant in Garín, Argentina, and then sent to Liomont Laboratories in Ocoyoacac, Mexico, for packaging and distribution.10mAbxience, “mAbxience enters into an agreement with AstraZeneca to produce a Covid-19 vaccine,” August 17, 2020, https://www.mabxience.com/mabxience-enters-into-an-agreement-with-astrazeneca-to-produce-covid-19-vaccine/.
There are, however, several chronic hurdles to expanding local production in Latin America. Vaccines are a highly complex product of advanced manufacturing. Most countries outside Argentina, Brazil, and Mexico do not yet possess the adequate infrastructure or sophistication required to produce or store vaccines at scale. Another key issue to consider is intellectual property (IP) protection, a major concern for multinational pharmaceutical companies. During the first months of the pandemic, a number of regional governments contemplated compulsory licensing measures out of understandable concerns around vaccine accessibility. While well-intentioned, such policies could disincentivize international vaccine makers and investors, who prefer to operate in a regulatory environment with robust patent enforcement.
As with other regions of the world, Latin America has reeled from the disruptions to global supply chains caused by COVID-19. In particular, overreliance on foreign medical supplies is fueling a regional drive toward greater self-sufficiency in pharmaceutical products. This is a warranted strategic ambition and welcome news for overstretched global capacity in the long run. Yet, scaling up regional production takes time and cannot singlehandedly address the ongoing emergency. In the short run, countries must also embrace another option to acquire lifesaving medicines and products: trade, which will be discussed in the next section.11While governments could sometimes gravitate toward a more national or regional view on vaccine access, industry tends to take a global approach. Manufacturing networks are usually set up in a globalized way to ensure the safest and fastest supply of a vaccine not only for Latin America, but also other regions around the globe. Most vaccine makers, including some with clinical trials in and supply agreements with Latin America, will plan on producing mostly outside the region.
In the short run, countries must also embrace another option to acquire lifesaving medicines and products: trade.
Over the long run, well-designed industrial, investment, and education policy support is vital to increasing the R&D and production capacity of vaccines, therapeutics, and medicines in Latin America. Value-added cooperation with key international firms brings in investment and generates positive externalities on local skills and knowledge. This, coupled with effective public-private coordination on IP issues, will benefit the regional innovation ecosystem which also encompasses many other high-value-add sectors.
- Trade
For much of the region with limited manufacturing capacity, trade will be the most realistic pathway to meeting domestic vaccine needs during the current crisis. Even for major vaccine producers in Latin America, trade is of paramount importance. Vaccines are a highly complex compound involving numerous ingredients and stages of production. Few countries in the world possess all the necessary specializations and basic materials to produce a competitive and fully “local” vaccine. A made-in-Latin-America vaccine could entail active pharmaceutical ingredients (APIs) from China or formulation development in India, as well as adjuvants from Chile processed in Sweden.12Thomas J. Bollyky and Chad P. Bown, “The Tragedy of Vaccine Nationalism,” Foreign Affairs, September/October 2020, https://www.foreignaffairs.com/articles/united-states/2020-07-27/vaccine-nationalism-pandemic. Rory Horner, “The world needs pharmaceuticals from China and India to beat coronavirus,” Conversation, May 25, 2020, https://theconversation.com/the-world-needs-pharmaceuticals-from-china-and-india-to-beat-coronavirus-138388. The import of intermediate goods is critical to the seamless production and assembly of final goods (vaccines) used for domestic consumption or export.
Given the globalized nature of vaccine manufacturing, governments in Latin America and beyond must ensure unimpeded trade flows across borders. Protectionist temptations can be hard to resist amidst global shortages, especially for countries with greater vaccine self-sufficiency. But these measures rarely pan out as desired and could result in dire regional and global consequences, including an unfortunate scenario of “vaccine nationalism.”13Bollyky and Bown, “The Tragedy.”
Given the globalized nature of vaccine manufacturing, governments in Latin America and beyond must ensure unimpeded trade flows across borders.
The breakdown of global trade in medical supplies earlier this year provided a fresh reminder of the still-present risks of protectionism. In March and April, for instance, eighty countries imposed export restrictions on medical supplies and equipment.14Andrea Shalal, “WTO report says 80 countries limiting exports of face masks, other goods,” Reuters, April 23, 2020, https://www.reuters.com/article/us-health-coronavirus-trade-wto/wto-report-says-80-countries-limiting-exports-of-face-masks-other-goods-idUSKCN2253IX. This included at least seven countries in Latin America and the Caribbean region, as well as the world’s top three suppliers [China, the United States, and the European Union (EU)],15Economic Commission for Latin America and the Caribbean (ECLAC), Restrictions on the export of medical products hamper efforts to contain coronavirus disease (COVID-19) in Latin America and the Caribbean, May, 2020, https://repositorio.cepal.org/bitstream/handle/11362/45511/2/S2000308_en.pdf. which collectively account for 68.2 percent of regional imports of these critical goods.16Banco Interamericano de Desarrollo, “Medidas aplicadas por los paises de ALC con impacto sobre comercio de bienes y servicios,” April 8, 2020, https://conexionintal.iadb.org/2020/04/08/medidas-aplicadas-por-los-paises-de-alc-con-impacto-sobre-comercio-de-bienes-y-servicios-2/. To protect lives and livelihoods, vicious cycles of commercial isolationism and retaliation must be avoided at all costs.
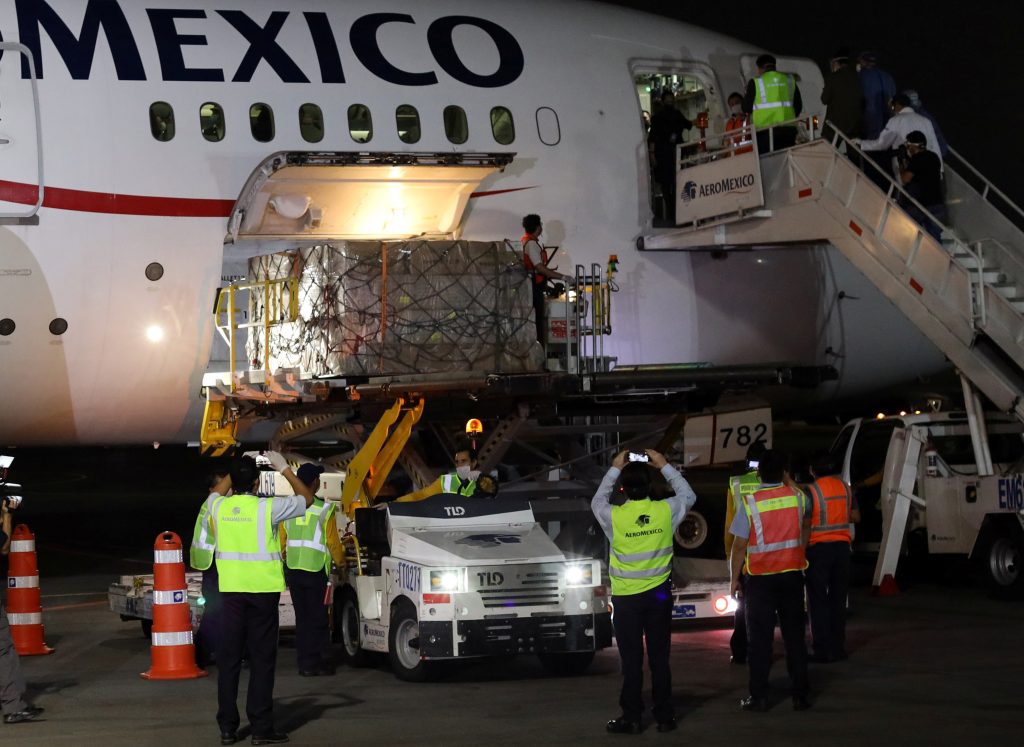
Aeromexico staff unloads a medical shipment arriving at the Benito Juarez International Airport. Given Latin America’s limited resources and local manufacturing capacities, engaging in international trade will be paramount to securing regional access to equipment, vaccines, and treatments for COVID-19. Picture taken on April 7, 2020. REUTERS/Carlos Carrillo
Specifically, Latin American and Caribbean policymakers can take actions to tackle shared challenges in trade on at least three levels. First, several viable quick wins exist at the national level. Governments should bring down two-way trade barriers on essential medical products and inputs, including import tariffs and export restrictions. Trade facilitation can reduce additional nontariff barriers through streamlined customs procedures and border crossings, electronic filing, expedited certifications and licensing, etc. Brazil, for instance, suspended anti-dumping and simplified administrative processes for import and export licensing of PPE and medical devices.17World Trade Organization, “How WTO Members Have Used Trade Measures to Expedite Access to COVID-19 Critical Medical Goods and Services,” information note, September 18, 2020, https://www.wto.org/english/tratop_e/covid19_e/services_report_16092020_e.pdf. Similar measures should be upheld to safeguard the trade of vaccines, as well as therapeutics, and other lifesaving products and services.
Second, international coordination between governments can further galvanize and amplify country-level actions. The Joint Ministerial Statement to ensure supply chain connectivity amidst the COVID-19 situation, initiated by Singapore and New Zealand in March, is an example to follow.18Singapore Ministry of Trade and Industry, Singapore concludes negotiations with New Zealand for Declaration on Trade in essential goods for Combating the COVID-19 Pandemic, press release, April 15, 2020, https://www.mti.gov.sg/Newsroom/Press-Releases/2020/04/Singapore-concludes-negotiations-with-New-Zealand-for-Declaration-on-Trade-in-essential-goods. As of July, ten other countries, including China, have joined the initiative, pledging their commitment to keep trade lines open for essential goods.19Singapore Ministry of Trade and Industry, Singapore concludes. Two Latin American countries, Chile and Uruguay, have also signed on.
Similarly, multilateral fora such as the Asia-Pacific Economic Cooperation (APEC) and regional integration processes such as Mercosur and the Pacific Alliance are other potential avenues to crowd in best trade practices. APEC members, including three Latin American countries, have issued at least three official declarations on trade facilitation.20Asia-Pacific Economic Cooperation, “Declaration on Facilitating the Movement of Essential Goods by the APEC Ministers Responsible for Trade (MRT),” accessed November 19, 2020, https://www.apec.org/Meeting-Papers/Sectoral-Ministerial-Meetings/Trade/2020_MRT/Annex-A. Asia-Pacific Economic Cooperation, “Statement on COVID-19 by APEC Ministers Responsible for Trade,” May 5, 2020, https://www.apec.org/Meeting-Papers/Sectoral-Ministerial-Meetings/Trade/2020_trade. Asia-Pacific Economic Cooperation, “2020 APEC High Level Meeting on Health and the Economy,” September 23, 2020, https://www.apec.org/Meeting-Papers/Sectoral-Ministerial-Meetings/Health/2020. The Pacific Alliance is playing a critical role in trade policy coordination in Latin America and internationally through its COVID-19 Action Plan and ASEAN-Pacific Alliance Work Plan.21Association of Southeast Asian Nations, “ASEAN and the Pacific Alliance to Forge Closer Relations in the Midst of COVID-19 Pandemic,” September 29, 2020, https://asean.org/asean-pacific-alliance-forge-closer-relations-midst-covid-19-pandemic/.
Third, collaboration between the public and private sectors is imperative. Delayed arrivals and departures of essential goods can be costly, especially for time-sensitive products like vaccines. Most vaccines are transported in refrigerated (or frozen) conditions and have limited room temperature shelf life, e.g., between two to twelve hours for Pfizer and Moderna’s COVID-19 vaccines.22The Moderna vaccine can withstand normal refrigeration conditions for up to thirty days; the Pfizer vaccine, only up to twenty-four hours. Zachary Brennan and Sarah Owermohle, “There are 2 effective Covid-19 vaccines. What’s next?” Politico, November 16, 2020, https://www.politico.com/states/new-york/city-hall/story/2020/11/16/there-are-2-effective-covid-19-vaccines-whats-next-1336557. Even before COVID-19 disruptions, in 2019, the average time to clear exports through customs in Latin America and the Caribbean region was eight days; the average time associated with border compliance for imports was 2.3 days.23Author’s calculations. World Bank. n.d. “World Bank Open Data.” Accessed November 19, 2020, https://data.worldbank.org/.
Collaboration between the public and private sectors is imperative.
Accelerating clearance can be achieved through efficient prioritization, nonintrusive inspection, digitization, etc. In addition, airports, seaports, and border authorities should work closely with logistics companies, vaccine producers, and various types of Authorized Economic Operators (importers, brokers, warehouses, and others). New requirements, processes, schedules, or contingent plans that may arise during the pandemic must be communicated clearly and promptly.
Another key area of public-private collaboration in trade is “hard” infrastructure. Enhanced interconnectivity can revitalize regional exports and intra-regional trade, benefitting pharmaceutical and many other supply chains in Latin America, making them more competitive. A 1 percent reduction in transport costs—achievable through infrastructure improvements—could boost overall manufacturing exports between 2 percent and 7.8 percent in Brazil, Chile, Colombia, Mexico, and Peru.24Mauricio Mesquita Moreira, Too Far to Export: Domestic Transport Costs and Regional Export Disparities in Latin America and the Caribbean, Inter-American Development Bank, 2013, https://publications.iadb.org/publications/english/document/Too-Far-to-Export-Domestic-Transport-Costs-and-Regional-Export-Disparities-in-Latin-America-and-the-Caribbean-(Executive-Summary).pdf. In the context of the COVID-19 pandemic, reduced shipping costs and time benefit not only regional vaccine acquisition and production, but in-country distribution of the vaccines and treatments.
Distribution and Access
With numerous agreements to acquire vaccines and treatments upon approval, governments must then face another difficult challenge: ensuring effective and equitable access to these lifesaving products in-country. Obstacles exist in at least two broad areas: distribution and access.
- Distribution
Latin America faces infrastructure and logistics challenges in distributing critical medical resources. These challenges are especially pronounced in rural and remote areas, including some of the hardest-hit parts of the region. Border towns and indigenous communities in the Amazon have reported high rates of COVID-19 cases and deaths. Logistic insufficiency has strained the already scarce medical supplies in these areas, aggravating outbreaks.25Santiago Torrado et al., “Las comunidades rurales en América Latina se enfrentan al avance del coronavirus,” El Pais, June 6, 2020, https://elpais.com/sociedad/2020-06-06/las-comunidades-rurales-en-america-latina-se-enfrentan-al-avance-del-coronavirus.html.
When vaccines become available, their distribution must comply with specific transportation and storage protocols to ensure safety and viability. Cold chain can be a particularly difficult and expensive requirement to satisfy. Most vaccines require a storage temperature of between two and eight degrees Celsius from manufacturer to patient. Select mRNA vaccines, such as the ones currently in trials by Pfizer, require ultra-cold storage—as low as -70 degrees Celsius.26Rebecca Weintraub, Prashant Yadav, and Seth Berkley, “A Covid-19 Vaccine Will Need Equitable, Global Distribution,” Harvard Business Review, April 2, 2020, https://hbr.org/2020/04/a-covid-19-vaccine-will-need-equitable-global-distribution. Ultra-cold transportation and storage will be a stress test on in-country logistic capabilities, challenging for most developing regions of the world. Inadequate basic infrastructure could cause additional wastage, e.g., blackouts that affect temperature-controlled equipment.27Lori Hinnant and Sam Mednick, “Vaccine storage issues could leave 3B people without access,” AP News, October 19, 2020, https://apnews.com/article/virus-outbreak-pandemics-immunizations-epidemics-united-nations-fc4c536d62c5ef25152884adb1c14168.
Following the 2015-2020 Regional Immunization Action Plan, Latin America and the Caribbean region has expanded cold-chain operations in recent years.28Pan American Health Organization, “Plan of Action on Immunization,” 54th Directing Council, 67th Session of the Regional Committee of WHO for Americas, September 30, 2015, https://www.paho.org/hq/dmdocuments/2015/CD54-7-e.pdf. Yet, bottlenecks remain. Large parts of the region are not equipped for downstream distribution at the unprecedented scale required to address the current health crisis.29DHL, “Delivering Pandemic Resilience,” white paper, September, 2020, https://www.dhl.com/content/dam/dhl/global/core/documents/pdf/glo-core-delivering-pandemic-resilience-2020.pdf. Distributing vaccines and other lifesaving products in a safe, efficient, and equitable manner will require additional investments and creative solutions.
The various trade facilitation measures that expedite imports (described in the previous section) will be the first step. Then, new partnerships with qualified operators of refrigerated transportation will be conducive to furthering in-country deployment, especially in the last mile. This should include supply chain leaders in other sectors—such as retail and consumer goods—capable of adapting current cold-chain operations to pharmaceutical needs while ensuring safety. Serving remote areas with limited infrastructure will require additional precision in demand planning and pooling. For instance, prioritizing clinics in relatively accessible locations (and schedules) can accelerate vaccine administration and maximize utilization, thus reducing pressure on storage and personnel.
Distributing vaccines and other lifesaving products in a safe, efficient, and equitable manner will require additional investments and creative solutions.
- Access
With acquisition and distribution plans in place for vaccines and treatments, Latin America and the Caribbean region must also ensure maximal utilization of these lifesaving products among populations. A patient-centric approach is critical to identifying specific hurdles in each country and designing effective countermeasures.
Supported by the Pan American Health Organization (PAHO) and its Revolving Fund, the Americas region has made significant progress in immunization, currently boasting one of the highest vaccine coverage levels in the world.30Pan American Health Organization, “Immunization,” accessed November 19, 2020, https://www.paho.org/en/topics/immunization. Many countries have carried out large-scale vaccinations, e.g., Brazil’s national immunization program (Programa Nacional de Imunizações) is able to vaccinate around three hundred million people per year.31Carla Maga Allan Santos Domingues, Antônia Maria Teixeira, and Sandra Maria Deotti Carvalho, Case Study: The Policy for the Introduction of New Vaccines in Brazil, SABIN Vaccine Institute, accessed November 19, 2020,https://www.sabin.org/sites/sabin.org/files/dominguesteixeiracarvalho_v2.pdf. However, considerable heterogeneity exists both across and within countries. At the subnational level, hard-to-reach areas tend to encounter greater resource and informational constraints to receive or properly administer vaccines, a challenge exacerbated by logistic hurdles mentioned earlier. At the national level, according to a 2018 survey, public skepticism about vaccines varied from between 3 percent to 5 percent of respondents in Argentina to between 15 percent and 17 percent in Peru.32Samantha Vanderslott, Bernadeta Dadonaite, and Max Roser, “Vaccination,” Our World in Data, last updated December, 2019, https://ourworldindata.org/vaccination.
Vaccine and overall medical hesitancy may derive from numerous factors: cultural norms, legislation, health literacy levels, personal experiences, among others. Awareness and educational campaigns can help mitigate some of these issues, especially in combating misinformation around vaccines, treatments, and the ongoing pandemic. Timely and accurate information sharing is key to enhancing public readiness for inoculation, as well as managing expectations. To this end, public and private sectors must cooperate and share medical updates, pricing, timelines, risks, and other information with each other. Together, they must better communicate the relevant information to the public.
Pricing, for instance, will be a fundamental determinant of patient access in many countries. A delicate balance must be struck between patient accessibility and commercial viability. Based on public sources, the expected market prices of some of the most advanced vaccine candidates as of October are: AstraZeneca/Oxford (between $3 and $4 per dose), Johnson & Johnson ($10), BioNTech/Pfizer ($39), and Moderna ($37).33Michael Peel et al., “How much will a Covid-19 vaccine cost?” Financial Times, October 22, 2020, https://www.ft.com/content/80f20d71-d7eb-4386-b0f2-0b19e4aed94d. Michael Erman and Ankur Banerjee, “U.S. to pay Pfizer, BioNTech $1.95 billion for COVID-19 vaccine,” Reuters, July 22, 2020, https://www.reuters.com/article/us-health-coronavirus-usa-pfizer/u-s-to-pay-pfizer-biontech-1-95-billion-for-covid-19-vaccine-idUSKCN24N1I9. Treatments are likely more expensive, with Gilead’s remdesivir—the first FDA-approved COVID-19 treatment for emergency use—possibly costing between $2,340 and $3,120 for a five-day cycle.34Matthew Herper, “Gilead announces long-awaited price for Covid-19 drug remdesivir,” STAT News, June 29, 2020, https://www.statnews.com/2020/06/29/gilead-announces-remdesivir-price-covid-19/. Important caveats should be taken into consideration: vaccine and treatment prices paid by consumers will vary and could be significantly lower, given likely government subsidies and/or insurance coverage. Colombia, for instance, has committed to providing free access to all COVID-19 vaccines acquired and distributed by the government.35Portafolio, “La vacuna contra la covid será gratuita para los colombianos,” November 11, 2020, https://www.portafolio.co/economia/la-vacuna-contra-la-covid-19-sera-gratuita-para-los-colombianos-546534. Generic production could also impact prices. For instance, Gilead voluntarily licensed remdesivir within several Latin American markets, enabling the licensees to set their own prices for the generic product they produce.36Gilead, “Voluntary Licensing Agreements for Remdesivir,” 2020, https://www.gilead.com/purpose/advancing-global-health/covid-19/voluntary-licensing-agreements-for-remdesivir.
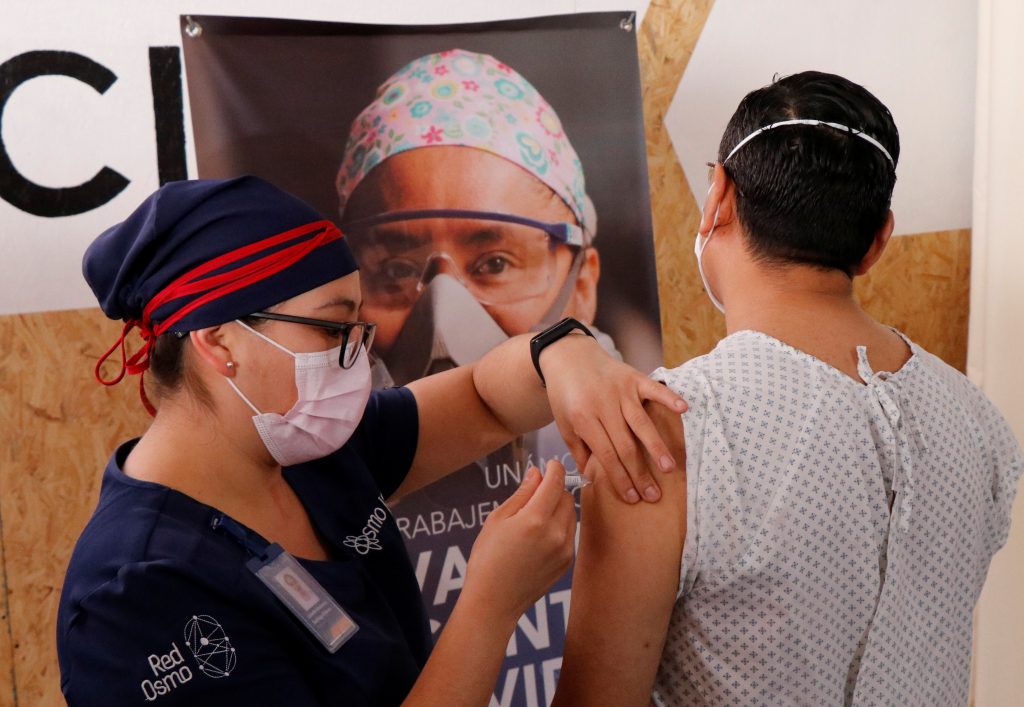
A volunteer receives an injection of a COVID-19 vaccine during a late stage-trial in Oaxaca, Mexico. Wide inoculation of vaccines in Latin America will depend on the region’s ability to overcome access-related challenges from deficient infrastructure to vaccine hesitancy. Picture taken on November 6, 2020. REUTERS/Jorge Luis Plata
As for timelines, recent estimates suggest some vaccine candidates, if approved, could be available for public use in the first half of 2021.37Pan American Health Organization, “PAHO information session for the press on COVID-19,” November 5, 2020, video, 1:02:05, https://www.youtube.com/watch?v=5KmZznYghCQ&ab_channel=PAHOTV. However, wide vaccination will take longer and can push well into 2022. Additional uncertainties could further impact the final cost and timeline of inoculation. Most vaccine front-runners as of late October require two doses separated by several weeks. How feasible is tracking and follow-up after the first injection?38Sarah Owermohle, “Historic vaccine race meets harsh reality,” Politico, October 27, 2020, https://www.politico.com/news/2020/10/27/vaccine-race-meets-harsh-reality-432964. Can vaccines generate long-lasting immunity, or would multiple rounds of vaccination be needed to ensure protection? While the humanitarian urgency has propelled several companies to sell at lower-than-usual prices, is this commercially viable over the medium/longer term? How might viral mutations affect the efficacy and safety of current vaccines? Countries must plan, budget, prepare, and communicate around these issues accordingly.
Relatedly, another key element of a well-organized vaccination strategy is the definition of “priority groups.” Given limited global availability of vaccines (and treatments) in the initial phase, shipments are expected to arrive in waves and in relatively small quantities. The first waves will be unable to meet overall demand. Countries must prioritize access by weighing needs and risks of population groups. There is a broad global consensus giving precedence to vulnerable populations and frontline medical workers, but classifications of each group may differ based on demographics, health conditions, and economic realities of each country. The WHO’s Prioritization Roadmap provides a useful framework for this analysis, including nuanced recommendations across different stages of vaccine availability and epidemiological scenarios.39World Health Organization, WHO SAFE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply, Version 1, October 20, 2020, https://www.who.int/docs/default-source/immunization/sage/covid/sage-prioritization-roadmap-covid19-vaccines.pdf?Status=Temp&sfvrsn=bf227443_2.
In sum, overcoming access and utilization challenges associated with COVID-19 vaccines necessitates a holistic plan for acquisition, distribution, and readiness. PAHO and the WHO, among other organizations, are actively providing technical and operational support for countries to smooth out vaccine introduction.40Pan American Health Organization, “Guidelines to Plan for COVID-19 Vaccine Introduction Version 1,” July 10, 2020, https://iris.paho.org/bitstream/handle/10665.2/52532/PAHOFPLIMCOVID-19200014_eng.pdf?sequence=1&isAllowed=y. World Health Organization, WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination, September 14, 2020, https://apps.who.int/iris/bitstream/handle/10665/334299/WHO-2019-nCoV-SAGE_Framework-Allocation_and_prioritization-2020.1-eng.pdf?ua=1. Some regional governments are already thinking ahead. Colombia, for instance, has committed $213 million to the global COVAX Facility to secure vaccines for ten million people, in addition to direct bilateral negotiations with pharmaceutical companies.41Julia Symmes Cobb, “As it hits 1 million coronavirus cases, Colombia prepares for vaccine,” Reuters, October 24, 2020, https://www.reuters.com/article/health-coronavirus-colombia/as-it-hits-1-million-coronavirus-cases-colombia-prepares-for-vaccine-idUSKBN2790LC. The Colombian government will also spend up to $78 million to facilitate in-country distribution and access of vaccines through improved transport, information campaigns, regional warehouse expansions, and personnel.
Overcoming access and utilization challenges associated with COVID-19 vaccines necessitates a holistic plan for acquisition, distribution, and readiness.
Other Issues
In addition to the four issues above (production, acquisition, distribution, and access), five other factors could shape the region’s COVID-19 vaccine readiness, as well as its transition towards a more sustainable and efficient post-COVID health model. These five factors are: funding, regulations, politics, multilateral cooperation, and non-COVID-19 conditions.
- Funding
Combating the health consequences of COVID-19 alone will require significant funding from governments in the region, from managing outbreaks and quarantines to acquiring and distributing eventual vaccines and treatments. As of May, the region was already spending 2.4 percent of GDP on health and economic responses (still below the 3.7 percent global average).42Cecilia Barria, “Coronavirus: los 10 paises que mas han gastado en enfrentar la pandemia y como se ubican los de America Latina,” BBC, May 18, 2020, https://www.bbc.com/mundo/noticias-52686453. In the short and medium term, increased health expenditures will be difficult to juggle in the context of tightening fiscal positions and a likely arduous economic recovery in Latin America. Most countries will not recover to pre-pandemic GDP levels until 2025.43Samuel Pienknagura, Jorge Roldós, and Alejandro Werner, “Pandemic Persistence Clouds Latin America and Caribbean Recovery,” IMFBlog, International Monetary Fund, October 22, 2020, https://blogs.imf.org/2020/10/22/pandemic-persistence-clouds-latin-america-and-caribbean-recovery/.
Over the long run, however, Latin America must confront and resolve chronic underinvestment in health systems, a critical preexisting vulnerability accentuated by COVID-19. Greater budgetary allocation toward health, combined with smart, efficient, and transparent spending, can boost the region’s public health and socioeconomic well-being, while better preparing it for the next pandemic. For example, scaling preventive care—as opposed to curative interventions—lowers medical bills for both patients and governments and simultaneously improves health outcomes. The same applies for transparent procurement of medical and pharmaceutical products, which can be prone to corruption and counterfeiting in the event of lax oversight. When appropriate, mobilizing private sector resources and well-designed public-private partnerships can complement and expand the traditional public provision of health goods and services.
Latin America must confront and resolve chronic underinvestment in health systems, a critical preexisting vulnerability accentuated by COVID-19.
- Regulations
Another way to alleviate resource constraints facing the region is through regulatory enhancements. Streamlined regulations in health, trade, and other areas will create efficiency gains needed to better cope with the historic urgency of the crisis. The objective is to strengthen health ecosystems, reducing the time and cost of pandemic response without sacrificing the quality of medical products and services. For instance, fast tracking (pre-)approvals, while ensuring safety, will accelerate production and distribution of successful vaccines. Many regulatory agencies in the region approve new drugs on an average minimum of twelve months.44Jerry Chapman, “Getting Drugs Approved In Mexico, Argentina, Colombia, And Peru,” REDICA Systems, November 14, 2020, https://govzilla.com/blog/2020/10/pharma-getting-drugs-approved-in-mexico-argentina-colombia-and-peru/#:~:text=Approval%20time%20for%20new%20products,as%20President%20in%20late%202018. During and even before the pandemic, steps are being taken to shorten this time frame. Colombia’s National Food and Drug Surveillance Institute (Instituto Nacional de Vigilancia de Medicamentos y Alimentos or INVIMA) was able to cut it down to eleven months from twenty-nine months.45Instituto Nacional de Vigilancia de Medicamentos y Alimentos, “Mejora en los tiempos de expedicion de registros sanitarios de medicamentos,” March 3, 2019, https://www.invima.gov.co/en/mejora-en-los-tiempos-de-expedicion-de-registros-sanitarios-de-medicamentos. Brazil’s National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária or ANVISA) has established faster regulatory pathways for approving diagnostics and medical devices.46FDA News, “Brazil’s ANVISA Expedites Approvals for COVID-19 Devices and IVDs,” May 7, 2020, https://www.fdanews.com/articles/196995-brazils-anvisa-expedites-approvals-for-covid-19-devices-and-ivds.
Regulatory convergence and collaboration across countries can generate additional quick wins. Harmonizing customs regulations and processes, from product-specific certifications and requirements (e.g., packaging and bottling) to classifications of essential versus nonessential goods, can boost intra-regional trade in medical products at this critical juncture. The pandemic has forced countries to temporarily lock down and reopen at different times and durations, causing disruptions in cross-border trade flows and travel. Improved regional coordination can help anticipate and mitigate the impact of such desynchronization on citizens and businesses. Together, these and other measures stand to benefit not only patients and the medical community, but other sectors of the economy that rely on international trade.
- Politics
While first and foremost a health crisis, the pandemic has had political ramifications across Latin America. Political realities and societal sentiments are inherently among key considerations of quarantine and reopening measures in each country. This has led to different policies and outcomes, as well as effects on leaders and institutions. In Chile, the pandemic postponed a constitutional referendum, whereas it accelerated presidential changes in Peru. Approval ratings of certain governments, such as in Brazil and Uruguay, have risen amid the pandemic, despite diverging policies and national contexts.47Clarín, “Seis meses de gobierno de Lacalle Pou en Uruguay: lo mejor y lo peor de una gestion ‘aprobada,’” August 30, 2020, https://www.clarin.com/mundo/uruguay-gobierno-lacalle-pou-aprobacion-popular-protestas-medio-pandemia_0_I9TApO4XA.html. Power dynamics between local and central governments are evolving, in part driven by pandemic-specific circumstances. The need for effective resource allocation and swift decision-making resulted in a relative weakening of local authorities in some cases, and a strengthening in others.
Protests of varying degrees broke out across the region, rooted in fresh discontent caused by COVID-19 or preexisting socioeconomic issues amplified by the pandemic. Neither set of underlying issues should be ignored. Systemic corruption, multidimensional inequality, and disinformation, for instance, could hinder the ongoing pandemic responses and the rapid, equitable access of eventual vaccines and treatments. Government capacity and credibility will make or break the rollout of these and other lifesaving products in Latin America. At this critical juncture, policymakers must also resist the temptation of party politics and unite against the common enemy, an unprecedented pandemic that does not discriminate based on politics.
The politicization of the pandemic is also playing out beyond national borders, impairing an already vulnerable and ailing international system. From the role of the WHO to cross-border medical donations and assistance, geopolitical competition and concerns have complicated a much-needed global coordination of efforts against the virus. Some frictions will likely linger during the vaccine phase.
A multi-stakeholder effort is needed to curb political and other distortions of science. As Latin American governments consider potential vaccines and treatments—whether from the United States, the EU, China, Russia, or elsewhere—the foremost considerations should be health and science-based: safety, efficacy, and transparency. Multilateral and nongovernmental organizations and initiatives can help provide valuable guidance in this regard. When in doubt, citizens and patients must turn to professionals and reputable health authorities for medical advice, instead of relying on unsubstantiated information. Social media platforms should continue to stem COVID-19-related disinformation and misinformation, especially in Latin America where unproven drugs and treatments have gained traction in numerous countries, at times abetted by politically motivated messaging.
- Multilateral cooperation
Multilateral coordination and assistance have been critical during the ongoing pandemic, from supporting outbreak control to mitigating the effects of famine and economic collapse. Such coordination will be equally important in a much-anticipated transition toward post-pandemic life, made possible by vaccines and treatments.
Promising progress has already been made through multilateral initiatives such as the COVAX Facility. The COVAX Facility aims to secure and equitably provide $2-billion-worth doses of COVID-19 vaccines to participating countries by the end of 2021. On the demand side, COVAX pools demand from countries and negotiates with manufactures on their behalf, thus ensuring affordable prices through scale. On the supply side, COVAX works with a diversified portfolio of eighteen vaccine candidates to increase the probability of success and availability.48World Health Organization, 172 countries and multiple candidate vaccines engaged in COVID-19 vaccine Global Access Facility, news release, August 24, 2020, https://www.who.int/news/item/24-08-2020-172-countries-and-multiple-candidate-vaccines-engaged-in-covid-19-vaccine-global-access-facility.
When the eventual vaccines first become available, manufacturing capacity will inevitably lag behind demand. Bidding wars and hoarding could cause global shortages and unequal access—as with PPE and ventilators at the beginning of the pandemic—with less resourceful countries bearing the brunt. The COVAX Facility will help mitigate these problems during this acute phase of demand-supply mismatch. All participating countries are expected to receive enough doses from COVAX to inoculate between 10 percent and 30 percent of their population (based on their own requests) over time. Most Latin American members opted for 20 percent or above.
As of November, one hundred and eighty-six economies had joined COVAX, including ninety-four higher-income economies (self-financed participants paying full prices) and ninety-two low and middle-income economies eligible to be subsidized by Gavi’s Advance Market Commitment (AMC).49World Health Organization, “WHO Director-General’s opening remarks at the media briefing on COVID-19,” October 19, 2020, https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—19-october-2020. COVAX, “Commitment.” For many countries in the latter group, COVAX represents their best chance at rapid and affordable access to safe and effective COVID-19 vaccines. Latin American and Caribbean participation has been enthusiastic from the outset. Twenty-two nations from the region have joined.50COVAX, “Commitment.” In addition, investors from Mexico and Panama have provided financial contribution to support the work of CEPI, which coordinates vaccine research, development, and manufacturing for the COVAX Facility.

In Mexico City’s Hospital Juarez de Mexico, this intensive care unit has been assigned to treat COVID-19 patients. COVID-19 has applied unprecedented pressure on Latin America’s health systems, affecting clinical attention to COVID and non-COVID conditions alike. Picture taken on October 29, 2020. REUTERS/Carlos Jasso
A truly global effort, the COVAX Facility is made possible by member countries, pharmaceutical companies, public and private sector donors (such as the EU and the Bill & Melinda Gates Foundation), and a network of partners (PAHO, the Inter-American Development Bank, the World Bank, various UN agencies, and many others). Going forward, additional support from non-member countries such as the United States could help ensure the continued success of the program, which needs an additional $5 billion in funding in 2021.51Gavi, Over US$ 2 billion raised to support equitable access to COVID vaccines with additional US$ 5 billion needed in 2021, press release, November 13, 2020, https://www.gavi.org/news/media-room/over-us-2-billion-raised-support-equitable-access-covid-vaccines-additional-us-5. Complementary efforts by national and subnational governments, multilateral organizations, and NGOs—from logistic support to combating disinformation—have the potential to enhance vaccine readiness in Latin America, thus laying the groundwork for successful vaccine introduction in-country.
- Non-COVID-19 conditions
The pandemic has overwhelmed health systems in dangerous yet under-studied ways. Seeing the pandemic from patients’ perspective, countries must refrain from considering action plans for COVID-19 control, treatment, and prevention in a silo. Inundated health facilities have impacted treatments, diagnostics, follow-ups, and overall attention to non-COVID-19 conditions. The delay of care disproportionately affects patients with noncommunicable diseases, who are also particularly vulnerable to COVID-19. This could be particularly detrimental to Latin America and the Caribbean region, where adult has obesity tripled since 1975, leading to heightened risks of cardiovascular illness and diabetes.52United Nations, “UN spotlights ‘explosive’ obesity rates, hunger in Latin America and Caribbean,” November 12, 2019, https://news.un.org/en/story/2019/11/1051211#:~:text=Since%201975%2C%20adult%20obesity%20in,by%20the%20UN%20on%20Tuesday. Missed in-person appointments and hospital visits could lead to a spike in these diagnostics in 2021 and 2022, potentially straining health systems once again.
Countries must refrain from considering action plans for COVID-19 control, treatment, and prevention in a silo.
Tackling patient access issues for all patients during and after the COVID-19 pandemic calls for a concerted effort among policymakers, business leaders, and the medical community, including patient advocacy groups. With the pandemic suppressing traditional health service delivery models, an unprecedented opportunity has emerged for collaborating around innovative solutions, such as telemedicine. However, mass telemedicine adoption, while timely, still faces foundational barriers in Latin America. Most of these challenges cannot be solved by the health industry or technology companies alone.
Digital infrastructure upgrades and improved connectivity will be fundamental, especially in rural and other underserved communities. Latin American hospitals and medical professionals can both accelerate and benefit from the widespread implementation of interoperable electronic health records, a telemedicine booster.53Pan-American Health Organization, “Electronic Health Records and Interoperability: Understanding Two Key Concepts for A Better Public Health Response,” Factsheet N.2, 2020, https://iris.paho.org/bitstream/handle/10665.2/52003/Factsheets-Digital_Health-EHR-Interoperability-eng.pdf?sequence=13. Legislators and regulators must address wide-ranging issues, from data and patient privacy to national and cross-border licensing of medical practitioners. This, coupled with other policy and commercial interventions, will ease practical concerns facing consumers/patients: confidence in the quality of virtual care, reimbursement for telehealth consultations, etc. As a whole, the region can extract important lessons from global experiences and efforts, such as best practices and discussions in the EU on telehealth.54A helpful survey of EU challenges in telemedicine: Sara Carrasqueiro et al., “Report on EU state of play on telemedicine services and uptake recommendations,” Joint Action to Support the eHealth Network, 2017, https://ec.europa.eu/health/sites/health/files/ehealth/docs/ev_20171128_co10_en.pdf. Despite vast cross-regional differences, many shared challenges and opportunities exist in this emerging space and could accelerate Latin America’s learning curve.
Conclusions and Recommendations
As the fight against COVID-19 evolves into a long haul, leaders in Latin America and around the world must mull over next steps. Sustained pandemic measures (testing, social distancing, and selective mobility restrictions) will remain the first line of defense against future infection waves for some time. Even when an effective vaccine becomes available, Latin America should prepare to live with the virus past 2021, given the time needed to ramp up global vaccine production and reach high levels of regional vaccination.
But getting ahead of the persistent virus will also require looking beyond the current phase of the pandemic. Countries should act decisively now to enhance readiness for potential vaccines and therapeutics, thus paving the way for a speedy post-pandemic recovery.
Countries should act decisively now to enhance readiness for potential vaccines and therapeutics, thus paving the way for a speedy post-pandemic recovery.
In this context, this issue brief brought to light nine key policy areas central to proactive vaccine and health planning, covering domestic and international factors from acquisition to access. Taking stock of the above, it prescribes a set of eight cross-cutting recommendations to help policymakers better manage the imminent challenges and strengthen health systems over the long term. These eight recommendations—summarized by the acronym PIER, with each letter representing two recommendations—consist of:
| The PIER Method | |
| P | Public-private collaboration will be critical across all areas of vaccine acquisition, production, distribution, access, etc. No single government or company can tackle these challenges alone given the unprecedented scale of the crisis and the solutions needed. Policymakers, regulators, industry leaders, patient groups, academia, multilateral organizations, and NGOs should continue to forge partnerships to protect citizens in the region. |
| P | Patient-centric approach must be at the heart of all solutions. Successful deployment of vaccines and treatments depends on policies and strategies that are attentive to patient pain points and expectations, which vary across and within countries. To the extent possible, health authorities should also begin surveying COVID-19’s effects on care for non-COVID-19 patients. In Latin America and the Caribbean, considerable backlogs of missed or delayed appointments, especially for noncommunicable diseases such as cancer and diabetes, can potentially put health systems under pressure once again after the pandemic. |
| I | Investment in health must increase. Over the long run, Latin American and Caribbean governments must increase health expenditure and strengthen complementary policies to support health systems. Regional countries currently spend 6.6 percent of GDP on health, whereas OECD countries average 8.8 percent.55OECD, “Health expenditure per capita and in relation to GDP,” accessed November 19, 2020, https://www.oecd-ilibrary.org/sites/6089164f-en/1/3/6/1/index.html?itemId=/content/publication/6089164f-en&_csp_=1ac29f0301b3ca43ec2dd66bb33522eb&itemIGO=oecd&itemContentType=book. Against the backdrop of short-term fiscal constraints, “spending better”—through improved efficiency and allocation—will be more achievable than “spending more” in most countries. |
| I | Innovation has helped the region to manage the pandemic and will deliver again in the future. For instance, innovative minds, solutions, and partnerships in Colombia caught international attention for building inexpensive, open-source ventilators amidst global shortages.56Juan Forero and Santiago Pérez, “Coronavirus Pandemic Prompts Race in Latin America to Build Cheaper Ventilators,” Wall Street Journal, April 23, 2020, https://www.wsj.com/articles/coronavirus-pandemic-prompts-race-in-latin-america-to-build-cheaper-ventilators-11587634202. A strengthened R&D ecosystem in Latin America appeals to domestic and foreign investment necessary for a robust post-pandemic recovery in health and beyond. Additionally, it sets the region up for long-term economic success while preparing it better for future pandemics. |
| E | Equity in vaccine and treatment access will be key to rapidly and extensively eradicating COVID-19. Failure to do so risks exacerbating the preexisting socioeconomic, gender, geographic, and other inequalities in the region. In the long run, health equity must be considered in broader terms. Equitable access to healthcare, insurance, nutrition, and technology (e.g., telemedicine) should contribute to a holistic effort to level the playing field for vulnerable populations. |
| E | Education and exchange of information benefit the whole of society. Education leads to greater health literacy and patient competency, thus empowering citizens against the pandemic and the accompanying infodemic. As lockdown fatigue kicks in, timely and consistent public sharing of science-based information related to COVID-19 (quarantines, treatments, vaccines, etc.) will become more important than ever. If done correctly, this is helpful for managing not just the virus but societal expectations. |
| R | Regional coordination and global collaboration are not mutually exclusive. Global problems require global solutions. Renewed multilateralism—such as the COVAX Facility—can help ensure efficient global production and acquisition of vaccines and other lifesaving products, as well as their fair and equitable distribution and access both across and within borders. But global collaboration should not come at the expense of regional integration, or vice versa. Latin American and Caribbean governments should reject the false dichotomy of global-versus-regional/national solutions, which emerged across the globe at the early stage of the pandemic characterized by PPE shortages and isolationism. |
| R | Regulatory enhancements and convergence must rise to the challenge. COVID-19 has taken a monumental toll on global health, but it has also triggered a series of equally extraordinary scientific breakthroughs. In a race against time, unprecedented R&D and partnerships compressed vaccine development processes from years to months. Policymakers and regulators must also proactively adapt to the circumstances. Where needed and while ensuring safety, government authorities must expedite internal (pre-)approvals and harmonize pertinent regulations across borders. A streamlined, predictable, and forward-thinking regulatory environment is vital to safeguarding public health in the immediate future, as well as fostering regional innovation and long-term competitiveness. |
Finally, the ongoing pandemic represents a unique learning opportunity and a possible health inflection point in the region, especially along the lines of the PIER recommendations above. Going back to the pre-pandemic normal will not be enough. Health systems need to be reinforced to prevent and better cope with the next pandemic. Despite great regional heterogeneity, perhaps the single biggest challenge and opportunity will be to transform the current health-focused policy momentum into longer-term actions and priorities. While most governments may not have the luxury to act on this at the moment, the Atlantic Council, through this and other writings, encourages governments—and relevant private, multilateral, and other stakeholders—to proactively (re)imagine a post-pandemic health agenda in the time ahead.
Image: The entrance of the intensive care unit where patients suffering from the coronavirus disease (COVID-19) are treated is pictured at Hospital Juarez de Mexico in Mexico City, Mexico October 29, 2020. REUTERS/Carlos Jasso